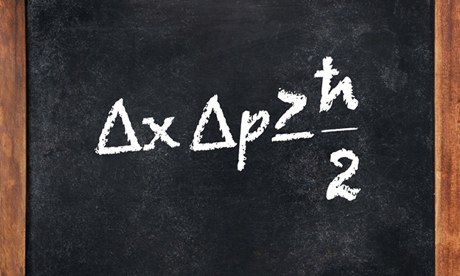Photoelectric effect is one of the initial phenomena which proved the quantization of light. It was discovered by Hertz in 1887 and Thomson in 1899 confirmed that it was electron that was responsible for photoelectric effect.
Einstein was the scientist behind more detail and elaborate explanation of photoelectric effect. He postulated his theory on photoelectric effect in 1905 and said that light consist of particles called photon. Each photon has an energy of and the linear momentum
where
is frequency of wave and
is the wavelength of the light.
When a beam of light of frequency is incident on a metal, each photon transmits all its
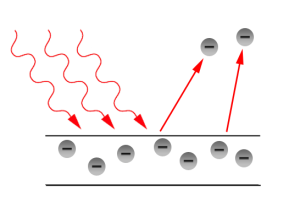 energy
energy to an electron neat the surface. In this case the photon is entirely absorbed by the electron. Thus the electron will absorb energy only in quanta of energy
and intensity of incident radiation doesn’t have any effect. There is always a minimum energy which is required to knock off electron from the metal and this energy is called work function
. If
is larger than
the electron will be knocked off and the electron will not jump if this condition is not satisfied. For
electron come out with kinetic energy with the maximum value
such that
where represents the kinetic energy of the electron leaving the material. This is Einstein photoelectric equation and gives explanation to photoelectric effect and is validated by experimental observation. From this equation we can see that the ejected electron’s kinetic energy increases linearly with the incident frequency
.
Here =
Here and is called threshold or cutoff frequency of the metal. This relation shows that no electrons are ejected from the metal unless
and the frequency of radiation is important rather than the intensity of the radiation to knock off electron. The kinetic energy of the knocked off electron is acquired from the excess energy
given by the incident radiation.