Radioactivity was first discovered by Henri Becquerel in 1896 when he noted that a uranium salt emitted radiation which showed its effect of a photographic plate. He further noticed that these radiation was spontaneous an didn’t require any excitation. The investigation to radioactivity was further done by scientist like Madame Curie, Piere curie and Rutherford. They show that radioactivity was show by heavier elements like thorium, radium, polonium, uranium etc and involved transformation of one element into another. Natural radioactivity occurs in heavier elements with atomic weight generally greater than 200 naturally. The element showing this phenomena are called radioactive elements. This transformation of atomic nucleus is accompanied by three different kind of rays called rays. The process of radioactivity is breaking up of nucleus spontaneously and is not affected by external factors like magnetic field, electric field, high pressure, high temperature etc.
Artificial radioactivity is also achieved in much lighter elements. This radioactive phenomena is achieved artificially by bombarding the element with particle, neutron, protons an other particles. The artificial radioactive elements usually have very short lifetime. They emit electrons, positrons, gamma rays etc during decomposition.
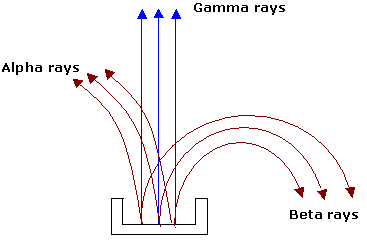
The presence of three distinct type of radiation can be shown easily. The alpha particle is attracted towards the negative charge proving that alpha particle is a positively charged, the beta particle is attracted towards the positive charge proving that it is negatively charged and gamma rays remains unaffected by electric field.
What are alpha particles
- They are helium nucleus with two proton and two neutron and xcess of two positive charges
- This velocity during radioactive emission is
- They ionize the gas through which they travel. Their ionization power is 10,000 times greater than gamma ray and 100 times greater than beta rays.
- Weakly effect photographic plate
- Produce fluorescence on substances like zinc sulphate or barium platinocyanide
- They are deflected by electric and magnetic field
- scattered by nucleus of heavy element like gold
What are beta particles
- have negative charge and mass equal to electron
- velocity of emitted beta particle range from 0.3c to 0.99c
- low ionization power and large range
- affect photographic plate
- Produce fluorescence on substances like zinc sulphate or barium platinocyanide
- deflected by electric and magnetic field
- greater penetrating power than alpha particles
What are Gamma rays
- they are electromagnetic waves with short wavelength. It range from 0.005A to 0.5A.
- travel with the speed of light
- produce fluorescence and affect a photographic plate.
- ionization power very weak
- more penetrating power than beta particles
- not effected by electromagnetic fields
- diffracted by crystals like x-rays.


