Somewhere around 440 BCE Democritus proposed that everything in the world was made up of tiny particles surrounded by empty space. And he even speculated that they vary in size and shape depending on the substance they compose. He called these particles “atomos,” Greek for indivisible. His ideas were opposed by the more popular philosophers of his day. Aristotle, for instance, disagreed completely, stating instead that matter was made of four elements: earth, wind, water and fire, and later scientists followed suit. Atoms would remain all but forgotten until 1808, when John Dalton sought to challenge Aristotelian theory.
Whereas Democritus’s atomism had been purely theoretical, Dalton showed that common substances always broke down into the same elements in the same proportions. He concluded that the various compounds were combinations of atoms of different elements, each of a particular size and mass that could neither be created nor destroyed. Atomic theory was now accepted by the scientific community, but the next major advancement would not come until nearly a century later with the physicist J.J. Thompson’s 1897 discovery of the electron. He showed atoms as uniformly packed spheres of positive charge matter filled with negatively charged electrons.
Thompson won a Nobel Prize in 1906 for his electron discovery, but his model of the atom didn’t stick around long. Ernest Rutherford, also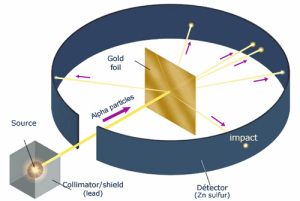 known as the father of the nuclear age while studying the effects of X-rays on gases, decided to investigate atoms more closely by shooting small, positively charged alpha particles at a sheet of gold foil. Under Thompson’s model, the atom’s thinly dispersed positive charge would not be enough to deflect the particles in any one place. The effect would have been like a bunch of tennis balls punching through a thin paper screen. But while most of the particles did pass through, some bounced right back, suggesting that the foil was more like a thick net with a very large mesh. Rutherford concluded that atoms consisted largely of empty space with just a few electrons, while most of the mass was concentrated in the center, which he termed the nucleus. The alpha particles passed through the gaps but bounced back from the dense, positively charged nucleus.
known as the father of the nuclear age while studying the effects of X-rays on gases, decided to investigate atoms more closely by shooting small, positively charged alpha particles at a sheet of gold foil. Under Thompson’s model, the atom’s thinly dispersed positive charge would not be enough to deflect the particles in any one place. The effect would have been like a bunch of tennis balls punching through a thin paper screen. But while most of the particles did pass through, some bounced right back, suggesting that the foil was more like a thick net with a very large mesh. Rutherford concluded that atoms consisted largely of empty space with just a few electrons, while most of the mass was concentrated in the center, which he termed the nucleus. The alpha particles passed through the gaps but bounced back from the dense, positively charged nucleus.
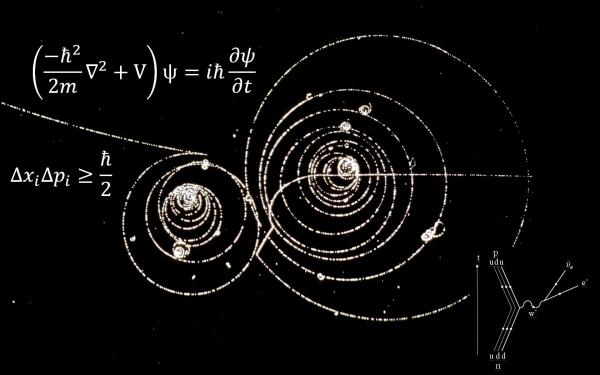
But the atomic theory wasn’t complete just yet. In 1913, another of Thompson’s students by the name of Niels Bohr expanded on Rutherford’s nuclear model. Drawing on earlier work by Max Planck and Albert Einstein he stipulated that electrons orbit the nucleus at fixed energies and distances, able to jump from one level to another, but not to exist in the space between. Bohr’s planetary model took center stage, but soon, it too encountered some complications. Experiments had shown that rather than simply being discrete particles, electrons simultaneously behaved like waves, not being confined to a particular point in space.
And in formulating his famous uncertainty principle, Werner Heisenberg showed it was impossible to determine both the exact position and speed of electrons as they moved around an atom. The idea that electrons cannot be pinpointed but exist within a range of possible locations gave rise to the current quantum model of the atom, a fascinating theory with a whole new set of complexities whose implications have yet to be fully grasped. Even though our understanding of atoms keeps changing, the basic fact of atoms remains.