In studying the florescence and phosphorescence of compounds irradiated with visible light, Bacquerel, in 1896, performed a crucial experiment which led to a deeper understanding of the properties of the nucleus of an atom. After illuminating some pieces of uranium-potassium sulfate with visible light, Bacquerel wrapped them in black paper and separated the package from a photographic plate by a piece of silver. After several hours’ exposure the photographic plate was developed and showed a blackening due to something that must have been emitted from the compound and was able to penetrate both the black paper and the silver.
Rutherford showed later that the emanations given off by uranium sulfate were capable of ionizing the air in the space between two oppositely charged metallic plate (an ionization chamber). The current registered by a galvanometer in series with the circuit was taken to be a measure of activity in the compound.
A systematic study of the activity of various elements and compounds led Mme. Curie to the conclusion that this activity was an atomic phenomena and by the methods of chemical analysis, she and her husband Pierre Curie found that “ionizing ability” or “activity” was associated not only with uranium but with two other elements that they discovered, radium and polonium. The activity of radium was found to be more than a million times that of uranium. Since the pioneer days of the Curies, many more radioactive substances have been discovered.
The activity of radioactive material may be easily shown to be the result of three different kinds of emanations. In 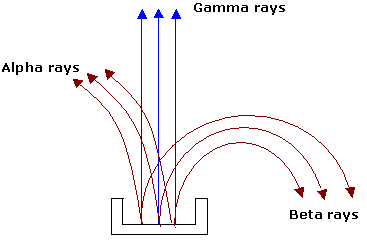 earlier experiments, a small piece of radioactive material is placed at the bottom of a long groove in a lead block. Some distance above the lead block a photographic plate is placed, and the whole apparatus is highly evacuated. A strong magnetic field is applied at right angles. After developing the plate, three distinct spots are found, one in the direct line of the groove in the lead block, one deflected to one side and one deflected to the other side. From the knowledge of the direction of the magnetic field, it is concluded that one of the emanations is positively charged (alpha particle), one is negatively charged (beta particle) and other is neutral (gamma rays).
earlier experiments, a small piece of radioactive material is placed at the bottom of a long groove in a lead block. Some distance above the lead block a photographic plate is placed, and the whole apparatus is highly evacuated. A strong magnetic field is applied at right angles. After developing the plate, three distinct spots are found, one in the direct line of the groove in the lead block, one deflected to one side and one deflected to the other side. From the knowledge of the direction of the magnetic field, it is concluded that one of the emanations is positively charged (alpha particle), one is negatively charged (beta particle) and other is neutral (gamma rays).
Further investigation showed that not all three emanations are emitted simultaneously by all radioactive substances. Some elements emit alpha particles, others emit beta particles, while gamma rays sometimes accompany one and sometimes the other. Furthermore, no simple macroscopic physical or chemical process such as raising or lowering the temperature, chemical combination with other non radioactive substance etc, could change or affect in any way the activity of a given sample. As a result, it was suspected from the beginning that radioactivity is a nuclear process and that the emission of a charged particle from the nucleus of an atom results in leaving behind a different atom, occupying a different place in the periodic table. In other words, radioactivity involves the transmutation of elements. The first measurement of the charge of the alpha particle used a device called a Geiger counter, still an important instrument of modern physics.